-
![Fighting resistant tumors with best-in-class inhibitors of casein kinase 1 (CK1)]()
Fighting resistant tumors with best-in-class inhibitors of casein kinase 1 (CK1)
CasInvent Pharma
About Us
CasInvent Pharma was established in 2020 as a spin-off of Masaryk University (MU) in cooperation with the investment partner i&i Prague, s.r.o. (Ltd). The mission of the company is to develop new therapeutic options for the treatment resistant tumors. The CasInvent platform is based on the use of proprietary, best in classhighly selective inhibitors of enzymes belonging to the Casein Kinase 1 (CK1) family that are responsible for the regulation of different cellular mechanisms leading to resistance for targeted therapies.
The CK1 inhibitors were discovered by the research groups of professors Vítězslav Bryja and Kamil Paruch at the Faculty of Science of MU, who have long-term expertise in the research areas of CK1 biology and chemistry of kinase inhibitors, respectively. CasInvent Pharma closely cooperates with these teams in advanced preclinical development of selected substances. The candidate compound is planned to enter clinical trials focused on the treatment of resistance Acute Myeloid Leukemia (AML) and solid tumor indications such as PDAC, TNBC, Melanoma or prostate cancer.

Leadership
Scientific Advisory
Investors






Our Science
Target: Casein kinase 1 (CK1)
- Serine/threonine kinases
- Isoforms α, γ1, γ2, γ3, δ and ε – different expression levels in different pathogeneses
- Implicated in cancer, AD, sleep disorders, obesity, opioid addiction
Goal: Development of highly potent and selective inhibitors of CK1
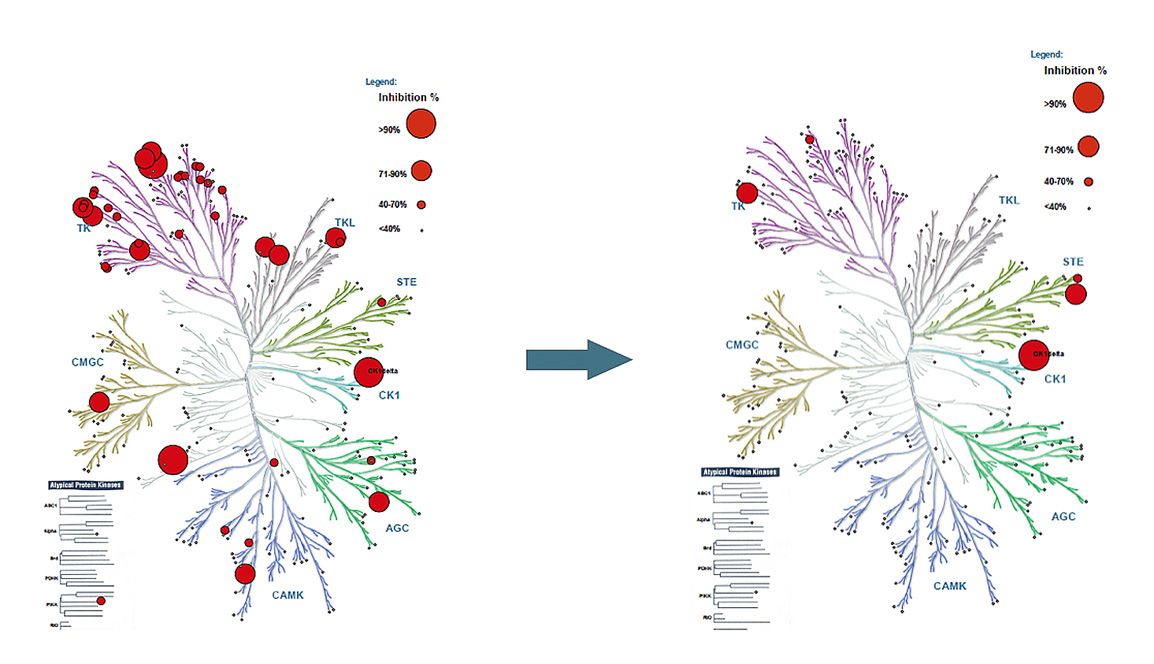
The small-molecule inhibitors developed by CasInvent Pharma are designed to target with high selectivity and activity individual isoforms of serine-threonine kinases from the Casein kinase 1 (CK1) family. In our research, we have focused on the isoforms CK1 δ, ε as therapeutic targets for the treatment of resistance in melanoma, TNBC and pancreatic cancer, and CK1 α for the treatment of acute myeloid leukemia (AML), with special focus on venetoclax resistance.
The development of the proprietary CK1 inhibitors is based on our studies.
Melanoma is a malignant neoplasm characterized by the uncontrolled growth of pigment-producing cells. Our investigation into the pathological cell signaling in melanoma has primarily concentrated on the regulators influencing cell migration and the interactions of melanoma cells with their surrounding microenvironment. This microenvironmental interaction often facilitates tumor cell survival, disease advancement, and evasion of therapeutic interventions.
Our findings revealed that selectively targeting CK1 δ and ε kinases in melanoma cells alone effectively impedes the migratory capacity of the malignant cells and disrupts their interactions with the protective microenvironment, leading to a significant delay in disease progression in murine models. Notably, the combination of CK1 inhibition with standard melanoma treatment, such as BRAF inhibitors, yielded enhanced, synergistic effects in vitro and a more robust therapeutic response in vivo in our established animal model of melanoma.
Targeting of the kinase CK1 α has been also recently recognized as an attractive therapeutic strategy for the treatment of AML . Similarly, in the realm of AML biology, this hematologic malignancy is marked by highly heterogeneous disease courses and an unclear pathogenesis. Our exploration of the pathological cell signaling in AML has predominantly focused on regulators governing cell migration and the interactions between leukemic cells and their supportive microenvironment. This microenvironmental support often contributes to the survival of tumor cells, disease progression, and resistance to therapeutic interventions. However, most of the published studies have used small-molecule inhibitors with low potency and poor kinase selectivity. Our recently identified series of molecules contains potent and highly selective inhibitors of CK1 α, with minimal off-target effects at therapeutic doses.
Background
- Research based on the activity of Pfizer compound PF-670462 on CK1
- New proprietary (patent pending) pharmacophores developed and optimized
- Developed compounds have better properties (selectivity, potency) than PF-670462
To date, more than 450 compounds have been prepared and tested:
- Small-molecule inhibitors (M < 400)
- ClogP = 2.0 - 4.5
- Efficient & modular synthesis, compounds can be rapidly prepared in gram quantities
- Well water soluble, compounds can be formulated as salts
Pipeline
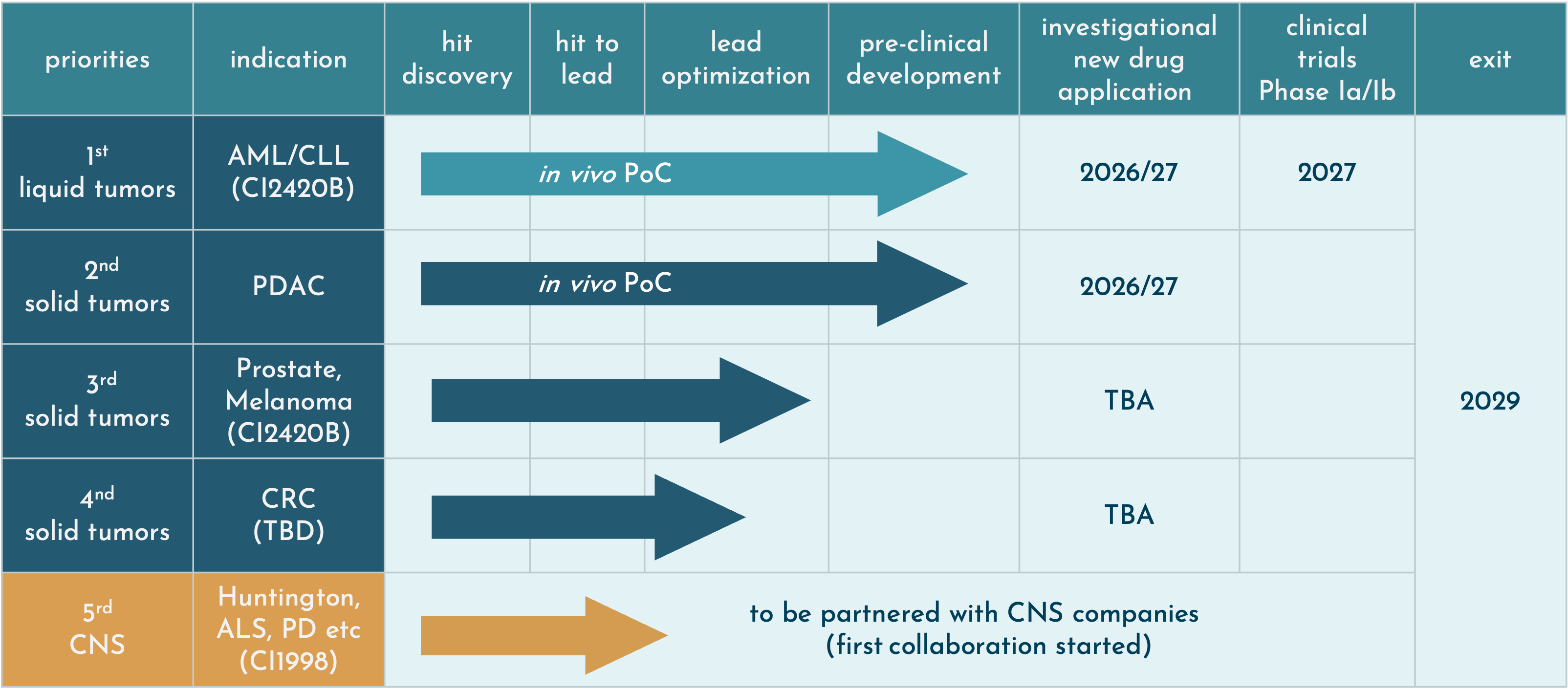
CasInvent Pharma is developing selective small molecule inhibitors of casein kinase 1(CK1). Other indications in the field of oncology are being studied, using the lead candidate as well as other molecules in our portfolio. CasInvent intends to advance its lead candidates to the clinical phase Ib.
The IP is subject to the exclusive sublicenceable worldwide license from the Masaryk University.
Publications
News
Corporate Headquarters
-
Komenského nám. 220/2
602 00 Brno
Czech Republic -
IČ: 09684221, DIČ: CZ09684221
-
Registration: Companies Register of the Regional Court in Brno, the Czech Republic, Ref. B 8460
General Information
© CasInvent